
Eighty-eight patients discontinued pembrolizumab due to persistent disease (n = 40), recurrent disease (n = 33), adverse events (AEs n = 10), achieved CR (n = 2), physician decision (n = 1), protocol violation (n = 1), and patient withdrawal (n = 1). Nineteen (48%) of the 40 responding patients maintained their response for 1 year or more.Īt an updated median follow-up of 21.1 months (range, 4.6-33.4), treatment was ongoing in 11 patients. Pembrolizumab elicited a CR rate of 41.2% (95% CI, 31.5-51.4), with a median duration of CR of 16.2 months (range, 0.0 to 26.8). Disease was assessed every 12 weeks, and those who did not have disease progression could receive treatment for up to 2 years. In cohort A, 96 patients were administered pembrolizumab 200 mg every 3 weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC, or disease progression. Key secondary endpoints were duration of response and safety. “The primary efficacy outcome measure was the proportion of patients achieving a complete response (complete eradication),” Balar said.
#Keynote 057 trial
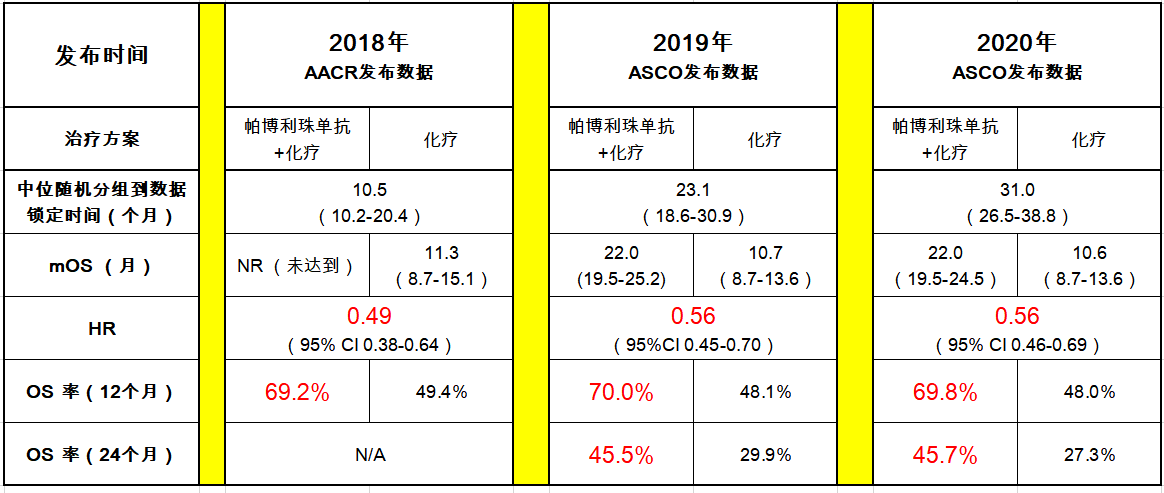
The multicenter, open-label, single-arm, multicohort phase II KEYNOTE-057 trial enrolled 102 patients with BCG-unresponsive, high-risk, NMIBC with CIS with or without papillary tumors who were ineligible for or did not undergo cystectomy. “With the approval of pembrolizumab, we have an effective option after BCG which will enable more patients to safely avoid a cystectomy.” KEYNOTE-057 Trial “A substantial proportion would outright refuse the surgery, and opt for other less effective treatments, which would then compromise their care,” acknowledged Balar. Prior to this approval, cystectomy was usually recommended to patients. Pembrolizumab is the first new drug approved for BCG-unresponsive high-risk NMIBC since 1998.
“This approval proves that systemic immunotherapy is active in a “localized” cancer process and has ushered the development of new treatments that could be combined with immunotherapy for the further benefit of patients with this disease,” said Balar. 2 The FDA recommended a dose of 200 mg every 3 weeks. The approval, which was based on findings from the phase II KEYNOTE-057 trial (NCT02625961), follows the 9-4 favorable vote in December 2019 from the FDA’s Oncologic Drugs Advisory Committee that supporting the approval of the new drug application for pembrolizumab. “While cystectomy is highly curative, there is a substantial impact on short and long-term quality of life with the surgery and high rates of complications as well, including an approximately 5% rate of mortality.”


Balar, MD, associate professor of medicine and director of the genitourinary medical oncology program at NYU Langone Health’s Perlmutter Cancer Center told ONCOLOGY. 1 “This is a major advance,” lead study investigator Arjun V. The FDA approved pembrolizumab (Keytruda) for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or chose to not undergo cystectomy.


 0 kommentar(er)
0 kommentar(er)
